
Public Notice on Pharmacovigilance by DGDA
📢 Public Notice on Pharmacovigilance by DGDA
Report Adverse Drug Reactions (ADR) or Adverse Events Following Immunization (AEFI)
If you experience any side effect or adverse reaction after taking any medicine or non-injectable vaccine, please report it to the Directorate General of Drug Administration (DGDA) by filling out the Yellow Card form.
You can submit your report through the following channels:
🔗 Online Submission:
👉 https://pvimsdashboard.com/yellow/card
✉️ Email:
👉 adrmcell.dgda@gmail.com
📮 Postal Address:
Director (Pharmacovigilance)
Directorate General of Drug Administration
Mohakhali, Dhaka-1212, Bangladesh
📞 PV Hotline:
👉 01728-349503
🌐 Website:
👉 www.dgda.gov.bd
👥 Who Can Report?
Doctors, nurses, pharmacists, patients, or caregivers — anyone can report suspected ADRs or AEFIs.
Latest News & events
 Public Notice on Pharmacovigilance by DGDA
Public Notice on Pharmacovigilance by DGDA NSU, UniMed sign MoU to improve pharmaceutical industry
NSU, UniMed sign MoU to improve pharmaceutical industry UniMed UniHealth Provides Financial Support to Language Movement Activist Ahmed Rafiq
UniMed UniHealth Provides Financial Support to Language Movement Activist Ahmed Rafiq Spread awareness about Kidney Health and encourage others to take care of their kidneys
Spread awareness about Kidney Health and encourage others to take care of their kidneys UniMed UniHealth Pharmaceuticals Limited awarded as highest VAT payer by NBR
UniMed UniHealth Pharmaceuticals Limited awarded as highest VAT payer by NBR ১০ অক্টোবর ২০২০, বিশ্ব মানসিক স্বাস্থ্য দিবস
১০ অক্টোবর ২০২০, বিশ্ব মানসিক স্বাস্থ্য দিবস Mental Health Awareness Campaign
Mental Health Awareness Campaign World Mental Health Day’2020
World Mental Health Day’2020 Launching of “Unifav” (Favipiravir)
Launching of “Unifav” (Favipiravir) Launching of “Nifev” (Nintedanib)
Launching of “Nifev” (Nintedanib) The Scientific Seminar on “Epilepsy & Its Management”
The Scientific Seminar on “Epilepsy & Its Management” The scientific seminar on “Update Management of COPD & SMART Therapy in Asthma” at Satkhira
The scientific seminar on “Update Management of COPD & SMART Therapy in Asthma” at Satkhira Annual Sales Conference 2019 of UniMed UniHealth Pharmaceuticals
Annual Sales Conference 2019 of UniMed UniHealth Pharmaceuticals Nizoder Anti-Dandruff Shampoo Environment Vanguard Award-2019
Nizoder Anti-Dandruff Shampoo Environment Vanguard Award-2019 Training on “Building Mega Brands”
Training on “Building Mega Brands” Training on “Driving Business Excellence through Leadership”
Training on “Driving Business Excellence through Leadership” The scientific seminar on “What makes Irbesartan standout than other antihypertensives.”
The scientific seminar on “What makes Irbesartan standout than other antihypertensives.” The Scientific seminar on “Management of Acute Migraine Headache & Update of PPIs”
The Scientific seminar on “Management of Acute Migraine Headache & Update of PPIs” International Neurology Seminar, 2019
International Neurology Seminar, 2019 The scientific seminar on “Acute Stroke & Neuro-Intervention”
The scientific seminar on “Acute Stroke & Neuro-Intervention” The scientific seminar on “Role of Levodopa in Parkinson’s Disease”
The scientific seminar on “Role of Levodopa in Parkinson’s Disease” The scientific seminar on “Stroke Masterclass”
The scientific seminar on “Stroke Masterclass” The scientific seminar on”Role of Bacterial Lysates for the Prophylaxis and Treatment of RTIs”
The scientific seminar on”Role of Bacterial Lysates for the Prophylaxis and Treatment of RTIs” The scientific seminar on “Role of Bacterial Lysates in Chronic Respiratory Diseases”
The scientific seminar on “Role of Bacterial Lysates in Chronic Respiratory Diseases” Introductory Ceremony of the 1st batch of MD Cardiology students in ICHRI
Introductory Ceremony of the 1st batch of MD Cardiology students in ICHRI The scientific seminar on “Role of Irbesartan in the management of Hypertension and Diabetic Nephropathy”
The scientific seminar on “Role of Irbesartan in the management of Hypertension and Diabetic Nephropathy” The scientific seminar on “Immunoadsorption or plasma exchange in treatment of autoimmune encephalitis: A pilot study”
The scientific seminar on “Immunoadsorption or plasma exchange in treatment of autoimmune encephalitis: A pilot study” The scientific seminar on “Updated Management of Dementia”
The scientific seminar on “Updated Management of Dementia” The scientific seminar on “A Comparison of intravenous Levetiracetam and Valproate for the treatment of refractory status epilepticus in children”
The scientific seminar on “A Comparison of intravenous Levetiracetam and Valproate for the treatment of refractory status epilepticus in children” The scientific seminar on “Are cerebrospinal fluid protein levels and plasma neutrophil/lymphocyte ratio associated with prognosis of Guillian Barre Syndrome?”
The scientific seminar on “Are cerebrospinal fluid protein levels and plasma neutrophil/lymphocyte ratio associated with prognosis of Guillian Barre Syndrome?” The scientific seminar on “Updated Management of Ischemic Stroke”
The scientific seminar on “Updated Management of Ischemic Stroke” The scientific seminar on “Updated Management of Parkinson’s Disease”
The scientific seminar on “Updated Management of Parkinson’s Disease” The scientific seminar on “Traumatic brain injury”
The scientific seminar on “Traumatic brain injury” The scientific seminar on “Management of movement disorder after stroke with movement therapy”
The scientific seminar on “Management of movement disorder after stroke with movement therapy” The scientific seminar on “Effectiveness of endoscopy surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy”
The scientific seminar on “Effectiveness of endoscopy surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy” The scientific seminar on “Comparative effectiveness of Rituximab and other initial treatment choices for multiple sclerosis”
The scientific seminar on “Comparative effectiveness of Rituximab and other initial treatment choices for multiple sclerosis” The scientific seminar on “Management of movement disorder after stroke with Hemiplegia”
The scientific seminar on “Management of movement disorder after stroke with Hemiplegia” The scientific seminar on “Anaesthesia for interventional neuroradiology”
The scientific seminar on “Anaesthesia for interventional neuroradiology” The scientific seminar on “Long-term Outcomes of Pediatric Ischemic Stroke in Adulthood”
The scientific seminar on “Long-term Outcomes of Pediatric Ischemic Stroke in Adulthood” The scientific seminar on “A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy”
The scientific seminar on “A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy” The scientific seminar on “Trombectomy 6 to 24 hour after stroke with mismatch between deficit and infarct.”
The scientific seminar on “Trombectomy 6 to 24 hour after stroke with mismatch between deficit and infarct.” The scientific seminar on “Different electrophysiological profile and treatment response in typical and atypical chronic inflammatory demyelinating polyneuropathy”
The scientific seminar on “Different electrophysiological profile and treatment response in typical and atypical chronic inflammatory demyelinating polyneuropathy” The scientific seminar on “A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy”
The scientific seminar on “A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy” The scientific seminar on “Management of hydrocephalus in children with posterior fossa tumors”
The scientific seminar on “Management of hydrocephalus in children with posterior fossa tumors” The scientific seminar on “Transcranial microsurgical and endoscopic endonasal cavernous sinus anatomy: a cadaveric study.”
The scientific seminar on “Transcranial microsurgical and endoscopic endonasal cavernous sinus anatomy: a cadaveric study.” The scientific seminar on “Management of Cerebral Vasculitis in indoor & Outdoor Patients”
The scientific seminar on “Management of Cerebral Vasculitis in indoor & Outdoor Patients” The scientific seminar on “Comparison of risk factor control in the year after discharge for ischemic stroke versus acute myocardial infarction”
The scientific seminar on “Comparison of risk factor control in the year after discharge for ischemic stroke versus acute myocardial infarction” The scientific seminar on “Aspirin resistance is associated with increased stroke severity and infarct volume”
The scientific seminar on “Aspirin resistance is associated with increased stroke severity and infarct volume” The scientific seminar on “Critical illness myopathy is frequent: accompanying neuropathy protracts ICU discharge.”
The scientific seminar on “Critical illness myopathy is frequent: accompanying neuropathy protracts ICU discharge.” The scientific seminar on “Effect of treating acute optic neuritis with bio-equivalent oral vs intravenous corticosteroids; a randomized clinical trial”
The scientific seminar on “Effect of treating acute optic neuritis with bio-equivalent oral vs intravenous corticosteroids; a randomized clinical trial” UniMed Pharma Celebrates Tk. One crore Milestone on Prazopress and Nexcital
UniMed Pharma Celebrates Tk. One crore Milestone on Prazopress and Nexcital The scientific seminar on “Management Update of CKD and AKI”
The scientific seminar on “Management Update of CKD and AKI” The scientific seminar on “Update Management of Hypertension”
The scientific seminar on “Update Management of Hypertension” The scientific seminar on “Updated Management on Angina Pectoris”
The scientific seminar on “Updated Management on Angina Pectoris” The scientific seminar on “Management of Dyslipidemia & Prevention of Atherosclerosis”
The scientific seminar on “Management of Dyslipidemia & Prevention of Atherosclerosis” The scientific seminar on “Role of Olmesartan in the management of Hypertension”
The scientific seminar on “Role of Olmesartan in the management of Hypertension” The scientific seminar on “ARDS: Recent Updates”
The scientific seminar on “ARDS: Recent Updates” WORKSHOP ON ECG: BASICS & BEYOND
WORKSHOP ON ECG: BASICS & BEYOND Honoring Ceremony, Cultural Program & Dinner of Mahesh Desai Urolithiasis Course at Dhaka Club by BAUS
Honoring Ceremony, Cultural Program & Dinner of Mahesh Desai Urolithiasis Course at Dhaka Club by BAUS Mahesh Desai Urolithiasis Course and Live Operative Workshop 2017 at BSMMU by BAUS
Mahesh Desai Urolithiasis Course and Live Operative Workshop 2017 at BSMMU by BAUS BAUS Monthly Scientific Seminar at BIRDEM
BAUS Monthly Scientific Seminar at BIRDEM 3rd Instructional Course on Urinary Incontinence 2017 & Certificate Giving Ceremony
3rd Instructional Course on Urinary Incontinence 2017 & Certificate Giving Ceremony 12th Instructional Course on BPH 2017 for GP & Certificate Giving Ceremony
12th Instructional Course on BPH 2017 for GP & Certificate Giving Ceremony 2nd Instructional Course on Sexual Medicine 2017 & Certificate Giving Ceremony
2nd Instructional Course on Sexual Medicine 2017 & Certificate Giving Ceremony 2nd Instructional Course on Urinary Incontinence 2017 & Certificate Giving Ceremony
2nd Instructional Course on Urinary Incontinence 2017 & Certificate Giving Ceremony The scientific seminar on “Management of Pneumonia”
The scientific seminar on “Management of Pneumonia” The scientific seminar on “The role of Bisoprolol in Cardiac Protection”
The scientific seminar on “The role of Bisoprolol in Cardiac Protection” The scientific seminar on “Management of Childhood Asthma”
The scientific seminar on “Management of Childhood Asthma” The scientific seminar on “Overview of Diabetic nephropathy”
The scientific seminar on “Overview of Diabetic nephropathy” Scientific Seminar on “Juvenile Periodontitis & Role of Chlorhexidine in Dental Care”
Scientific Seminar on “Juvenile Periodontitis & Role of Chlorhexidine in Dental Care” Scientific Seminar on Smart Therapy in Asthma & COPD Management-What’s new?
Scientific Seminar on Smart Therapy in Asthma & COPD Management-What’s new? Scientific seminar on “Chikungunya”
Scientific seminar on “Chikungunya” Scientific Seminar on “Overactive Bladder- Expert Master Class”
Scientific Seminar on “Overactive Bladder- Expert Master Class” 11th Instructional Course on BPH 2017 for GP & Certificate Giving Ceremony
11th Instructional Course on BPH 2017 for GP & Certificate Giving Ceremony 1st Instructional Course on Sexual Medicine 2017 & Certificate Giving Ceremony
1st Instructional Course on Sexual Medicine 2017 & Certificate Giving Ceremony Scientific Seminar on ‘Smart therapy in Asthma & COPD management – what’s new?’
Scientific Seminar on ‘Smart therapy in Asthma & COPD management – what’s new?’ Closing Ceremony of Sheikh Rasel Smrity Intra-sports Competition & Cultural Week 2017
Closing Ceremony of Sheikh Rasel Smrity Intra-sports Competition & Cultural Week 2017 Sheikh Rasel Smrity Intra-sports Competition & Cultural Week 2017
Sheikh Rasel Smrity Intra-sports Competition & Cultural Week 2017 SASSM School Workshop at Dhaka Club
SASSM School Workshop at Dhaka Club AGM of BAUS & Iftar Mahfil 2017
AGM of BAUS & Iftar Mahfil 2017 The scientific seminar on “Overview on Non-Invasive Ventilation”
The scientific seminar on “Overview on Non-Invasive Ventilation” The scientific seminar on “Dosing of respiratory Drugs in Chronic Kidney Disease (CKD)”
The scientific seminar on “Dosing of respiratory Drugs in Chronic Kidney Disease (CKD)” Iftar Party of Urology Dept., SSMCH
Iftar Party of Urology Dept., SSMCH Iftar Party of Urology Dept., DMCH
Iftar Party of Urology Dept., DMCH Iftar Party of Urology Dept., BSMMU
Iftar Party of Urology Dept., BSMMU The Scientific Seminar on “Updates on Chronic Pain Management”
The Scientific Seminar on “Updates on Chronic Pain Management” The Scientific Seminar on “Total Hip Arthroplasty”
The Scientific Seminar on “Total Hip Arthroplasty” The Scientific Seminar on “Updates on Chronic Pain Management”
The Scientific Seminar on “Updates on Chronic Pain Management” The Scientific Seminar on “Basic Principle of Ilizarov Technique”
The Scientific Seminar on “Basic Principle of Ilizarov Technique” Book giving ceremony in Pain Medicine Dept., BSMMU
Book giving ceremony in Pain Medicine Dept., BSMMU Workshop on “Ilizarov technic in Orthopedic Trauma”
Workshop on “Ilizarov technic in Orthopedic Trauma” The Scientific Seminar on “Pain Management in Cancer patient (Recent Updates)”
The Scientific Seminar on “Pain Management in Cancer patient (Recent Updates)” The Scientific Seminar on “Management of Cancer pain”
The Scientific Seminar on “Management of Cancer pain” The Scientific Seminar on “Kidney Transplantation – Why, When & How?” at Tangail
The Scientific Seminar on “Kidney Transplantation – Why, When & How?” at Tangail Course on Nuclear Studies in Urology at BSMMU
Course on Nuclear Studies in Urology at BSMMU 1st Instructional Course on Urinary Incontinence 2017 & Certificate Giving Ceremony
1st Instructional Course on Urinary Incontinence 2017 & Certificate Giving Ceremony CME on “Update Management of BPH” at Feni Sadar Hospital
CME on “Update Management of BPH” at Feni Sadar Hospital The Scientific Seminar on “Sylhet Symposium-Sexual Medicine Update” by SASSM
The Scientific Seminar on “Sylhet Symposium-Sexual Medicine Update” by SASSM CME on “Evaluation and Management of LUTS” at Mugda Medical College & Hospital
CME on “Evaluation and Management of LUTS” at Mugda Medical College & Hospital Scientific Presentation on “Updated Management on Overactive Bladder” at NIKDU
Scientific Presentation on “Updated Management on Overactive Bladder” at NIKDU 3rd Hands-on Training on Urological Ultrasound for 6 weeks
3rd Hands-on Training on Urological Ultrasound for 6 weeks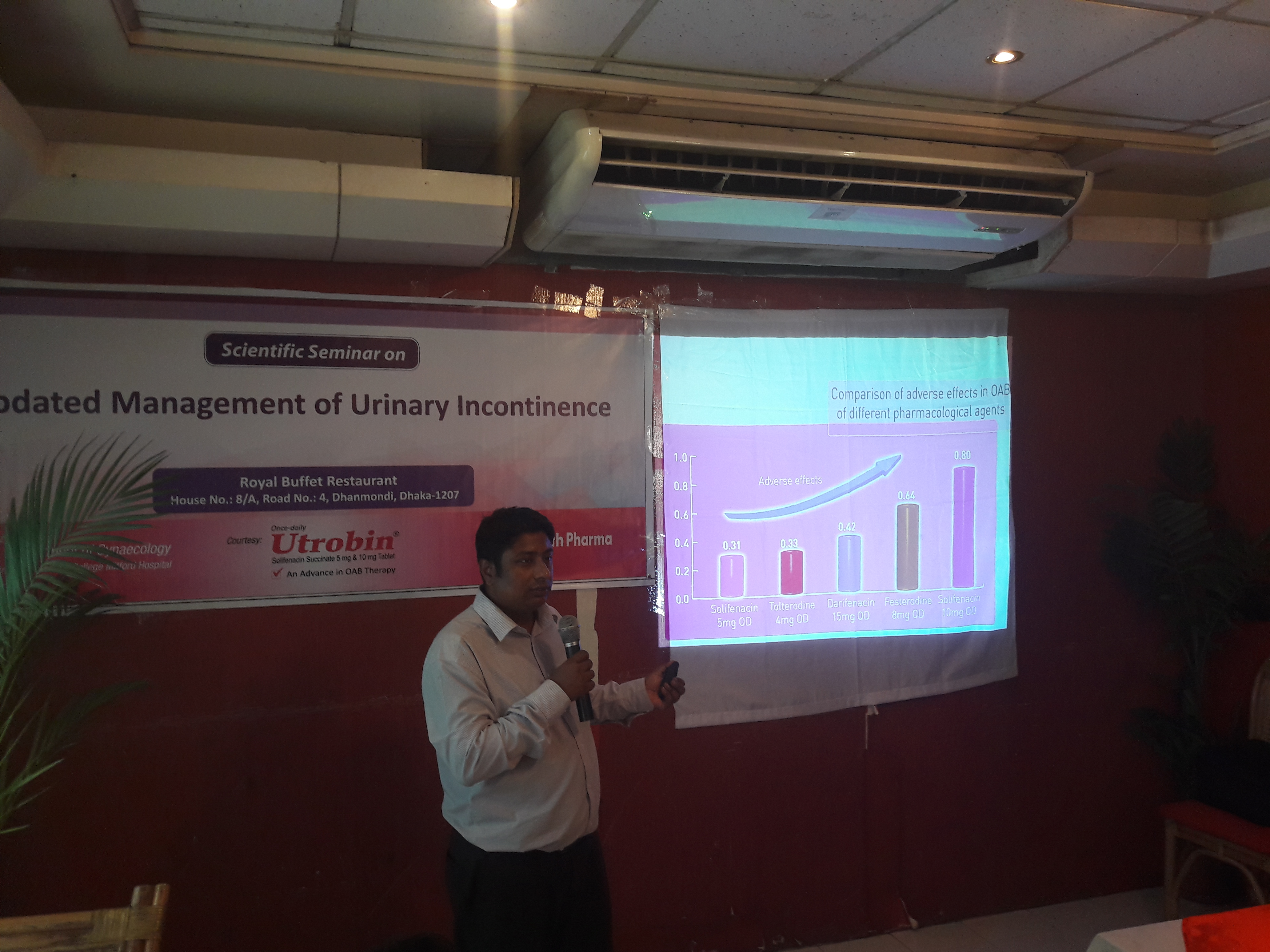 CME on “Updated Management of Urinary Incontinence & Case study Presentation”
CME on “Updated Management of Urinary Incontinence & Case study Presentation” Observing the “International Fistula Day 2017” & Scientific Seminar on Obstetric Fistula
Observing the “International Fistula Day 2017” & Scientific Seminar on Obstetric Fistula The scientific seminar on “Childhood Tuberculosis (An Overview)”
The scientific seminar on “Childhood Tuberculosis (An Overview)” The scientific seminar on “An Update of Asthma Management”
The scientific seminar on “An Update of Asthma Management” 10th Instructional Course on BPH 2017 for GP & Certificate Giving Ceremony
10th Instructional Course on BPH 2017 for GP & Certificate Giving Ceremony Monthly Seminar on “Carcinoma Prostate and Recent Advanced”
Monthly Seminar on “Carcinoma Prostate and Recent Advanced” The Scientific Seminar on “Bladder Outflow Obstruction”
The Scientific Seminar on “Bladder Outflow Obstruction” The Scientific Seminar on “Laparoscopy in Urology”
The Scientific Seminar on “Laparoscopy in Urology” 19th Annual General Meeting of BSSP and Logo Inauguration of 8th SARPS
19th Annual General Meeting of BSSP and Logo Inauguration of 8th SARPS Round Table Discussion on “Role of Tramadol in Post-operative pain management”
Round Table Discussion on “Role of Tramadol in Post-operative pain management” The Scientific Seminar on “Joint Replacement-Past/ Present/ Future”
The Scientific Seminar on “Joint Replacement-Past/ Present/ Future” The Scientific Seminar on “Intramedullary Nailing”
The Scientific Seminar on “Intramedullary Nailing” The Scientific Seminar on “Updates on Chronic Pain Management”
The Scientific Seminar on “Updates on Chronic Pain Management” The Scientific Seminar on ” Management of Chronic Pain”
The Scientific Seminar on ” Management of Chronic Pain” Round Table Discussion on ” Overview of Cancer Pain Management and Role of Oral Morphine in advanced cancer pain”
Round Table Discussion on ” Overview of Cancer Pain Management and Role of Oral Morphine in advanced cancer pain” Seminar on “Continuous Professional Development Program”
Seminar on “Continuous Professional Development Program” The Scientific Seminar on “Updated Management of Chronic Low Back Pain”
The Scientific Seminar on “Updated Management of Chronic Low Back Pain” Cancer Awareness Program
Cancer Awareness Program International CME on “Managing Lupus Nephritis and Adverse Drug Reactions”
International CME on “Managing Lupus Nephritis and Adverse Drug Reactions” Celebration of World Asthma Day 2017
Celebration of World Asthma Day 2017 Psychiatry Focus Group Meeting 2017- SEAL
Psychiatry Focus Group Meeting 2017- SEAL The scientific seminar on “Non Alcoholic Fatty Liver Disease- An Emerging Health Problem Nowadays”
The scientific seminar on “Non Alcoholic Fatty Liver Disease- An Emerging Health Problem Nowadays” CME on “Updated Management of Urinary Incontinence”
CME on “Updated Management of Urinary Incontinence” The scientific seminar on “Urological Problems in Female”
The scientific seminar on “Urological Problems in Female” Symposium on “Overactive Bladder”
Symposium on “Overactive Bladder” The scientific seminar on “Updated Management of Urinary Incontinence in Women”
The scientific seminar on “Updated Management of Urinary Incontinence in Women” The scientific seminar on “Update Management of Hypertension”
The scientific seminar on “Update Management of Hypertension” The scientific seminar on “The role of Irbesartan in the management of Diabetic Nephropathy & Hypertension”
The scientific seminar on “The role of Irbesartan in the management of Diabetic Nephropathy & Hypertension” The scientific seminar on “ECG MASTERCLASS”
The scientific seminar on “ECG MASTERCLASS” THE GREGARIOUS wins UniMed UniHealth Premier Basketball League 2017
THE GREGARIOUS wins UniMed UniHealth Premier Basketball League 2017 The scientific seminar on “Management of Systemic Hypertension”
The scientific seminar on “Management of Systemic Hypertension” The scientific seminar on “Hypertension & its Management”
The scientific seminar on “Hypertension & its Management” Observing World Kidney Day 2017
Observing World Kidney Day 2017 UniMed UniHealth sponsored Premier Basketball League 2017
UniMed UniHealth sponsored Premier Basketball League 2017 Round Table Meeting on “Updates on ACS Management”
Round Table Meeting on “Updates on ACS Management” Observing “World Hearing Day 2017” & Scientific Seminar on “Hearing Loss”
Observing “World Hearing Day 2017” & Scientific Seminar on “Hearing Loss” The scientific seminar on “Anti-Platelet Drugs”
The scientific seminar on “Anti-Platelet Drugs” The scientific seminar on “Refractory & Resistant Hypertension – A Brief Review”
The scientific seminar on “Refractory & Resistant Hypertension – A Brief Review” Free pain camp at Bhola
Free pain camp at Bhola The scientific seminar on “Persistent Chronic Pain Syndrome: Act Fast”
The scientific seminar on “Persistent Chronic Pain Syndrome: Act Fast” The scientific seminar on “Management of Vitiligo”
The scientific seminar on “Management of Vitiligo” The scientific seminar on “Management of Dandruff & SD”
The scientific seminar on “Management of Dandruff & SD” Training on “Brand Management”
Training on “Brand Management” The scientific seminar on “Management of Acne”
The scientific seminar on “Management of Acne” Annual Sales Meeting 2016 of UniHealth Pharma Respiratory ENT
Annual Sales Meeting 2016 of UniHealth Pharma Respiratory ENT Annual Business Meeting of JANSSEN
Annual Business Meeting of JANSSEN The scientific seminar on “Role of Irbesartan in the Management of Diabetic Nephropathy & Hypertension”
The scientific seminar on “Role of Irbesartan in the Management of Diabetic Nephropathy & Hypertension” Annual Sales Meeting 2016 of UniMed Pharma
Annual Sales Meeting 2016 of UniMed Pharma Annual meeting 2016 of Bioderma group held on 1st January 2017
Annual meeting 2016 of Bioderma group held on 1st January 2017 The scientific seminar on “Management of Psoriasis”
The scientific seminar on “Management of Psoriasis” Launching ceremony of activities of ACC (American College of Cardiology) in Bangladesh
Launching ceremony of activities of ACC (American College of Cardiology) in Bangladesh Symposium on ‘Management of patients with type-2 diabetes and mild to moderate renal impairment’
Symposium on ‘Management of patients with type-2 diabetes and mild to moderate renal impairment’ The scientific seminar on “Management of Dandruff & SD”
The scientific seminar on “Management of Dandruff & SD” The scientific seminar on “Management of Psoriasis”
The scientific seminar on “Management of Psoriasis” The scientific seminar on “Management of Psoriasis”
The scientific seminar on “Management of Psoriasis” The scientific seminar on “Management of Psoriasis”
The scientific seminar on “Management of Psoriasis” The scientific seminar on “Update Management of Hypertension Based on JNC 8”
The scientific seminar on “Update Management of Hypertension Based on JNC 8” The scientific seminar on “Role of Olmesartan in the management of Hypertension”
The scientific seminar on “Role of Olmesartan in the management of Hypertension” The scientific seminar on “Role of Irbesartan in the management of Diabetic Nephropathy & Hypertension”
The scientific seminar on “Role of Irbesartan in the management of Diabetic Nephropathy & Hypertension” Observing World Kidney Day 2016
Observing World Kidney Day 2016 The scientific seminar on “Update Management of Hypertension in CKD patients”
The scientific seminar on “Update Management of Hypertension in CKD patients” The scientific seminar on “The role of Irbesartan in the management of Diabetic Nephropathy & Hypertension”
The scientific seminar on “The role of Irbesartan in the management of Diabetic Nephropathy & Hypertension” The scientific seminar on “The role of Hypertension in Chronic Kidney Diseases”
The scientific seminar on “The role of Hypertension in Chronic Kidney Diseases” Scientific Seminar on “World Alzheimer’s Day 2015”
Scientific Seminar on “World Alzheimer’s Day 2015” FDA approve new drug to treat high cholesterol
FDA approve new drug to treat high cholesterol Coffee may harm cardiovascular health for young adults with mild hypertension
Coffee may harm cardiovascular health for young adults with mild hypertension Antibiotic use may raise risk of type 2 diabetes
Antibiotic use may raise risk of type 2 diabetes The scientific seminar on “The role of Irbesartan in the Management of Diabetic Nephropathy and Hypertension”
The scientific seminar on “The role of Irbesartan in the Management of Diabetic Nephropathy and Hypertension” Scientific Seminar on Lipid Managment Guidline on the basis of ATP 1V
Scientific Seminar on Lipid Managment Guidline on the basis of ATP 1V Seminar on “ SLEEP WELL : LIVE WELL” on the occasion of World Sleep Day 2015
Seminar on “ SLEEP WELL : LIVE WELL” on the occasion of World Sleep Day 2015 Reduced heart function tied to raised risk of dementia, Alzheimer’s
Reduced heart function tied to raised risk of dementia, Alzheimer’s What Do Smartphones Do to the Brain?
What Do Smartphones Do to the Brain? 10 Interesting Medical Facts of Human Body
10 Interesting Medical Facts of Human Body Annual Sales Conference 2014 of UniMed UniHealth
Annual Sales Conference 2014 of UniMed UniHealth Scientific Seminar on Role of Acetylcysteine
Scientific Seminar on Role of Acetylcysteine Specialist panel meeting on ATP-IV
Specialist panel meeting on ATP-IV Diabetes & Endocrine Conference
Diabetes & Endocrine Conference Scientific Seminar On “Management of Sexual Dysfunction”
Scientific Seminar On “Management of Sexual Dysfunction” Scientific Seminar On “Benign Paroxysmal Positional Vertigo: An Approach to Diagnosis & Management”
Scientific Seminar On “Benign Paroxysmal Positional Vertigo: An Approach to Diagnosis & Management” UniHealth Pharma organized a meeting with South Asian Society
UniHealth Pharma organized a meeting with South Asian Society Scientific Seminars On “BPH Management”
Scientific Seminars On “BPH Management” Health Tip: When Your Child Is Afraid to Sleep
Health Tip: When Your Child Is Afraid to Sleep Health Tip: Soothing Psoriasis on the Feet
Health Tip: Soothing Psoriasis on the Feet Kids With Autism Have Extra Brain Connections
Kids With Autism Have Extra Brain Connections NSAIDs may halve breast cancer recurrence in overweight women
NSAIDs may halve breast cancer recurrence in overweight women Coronary calcium predicts heart disease risk in patients with chronic kidney disease
Coronary calcium predicts heart disease risk in patients with chronic kidney disease Do colds increase the risk of stroke in children?
Do colds increase the risk of stroke in children? Exercise may protect older women from irregular heartbeat
Exercise may protect older women from irregular heartbeat Fenofibrate Effective for Women and Men With T2DM
Fenofibrate Effective for Women and Men With T2DM A New Easy DPP-4 Inhibitor Linagliptin for the management of 2 diabetes
A New Easy DPP-4 Inhibitor Linagliptin for the management of 2 diabetes The Role of ARB’s in the management of Hypertension
The Role of ARB’s in the management of Hypertension Management of Erectile Dysfunction
Management of Erectile Dysfunction
